Lumos Diagnostics (ASX:LDX) Update
THE Milestones strengthen Ahead of the CLIA Decision
General advice only – prepared for Wholesale/Sophisticated/Professional Investors. See full disclaimers below.
Lumos Diagnostics (ASX:LDX) sits right at the intersection of a global healthcare problem and a simple, scalable solution.
Antibiotic resistance is one of the biggest threats to modern medicine. GPs are still handing out antibiotics like confetti. Not because they want to, but because it’s often the safest guess when they can’t tell if an infection’s bacterial or viral.
Lumos’ FebriDx test changes that equation. It’s a 10-minute, finger-prick test that gives doctors a clear answer on the spot. No fancy machines. Just results.
The potential market is enormous. If Lumos secures a US CLIA waiver, FebriDx will move from laboratories into 270,000 point-of-care clinics, unlocking a US$1 billion market and transforming how infections are diagnosed.
Crucially, Lumos already has a US$317 million distribution agreement with PHASE Scientific. The deal depends on CLIA waiver approval. If it isn’t granted, there’s still a guaranteed base of about US$25 million. Either way, Lumos secures meaningful minimum purchase commitments and a strong revenue base, with even greater upside if the waiver lands.
Over the past few months, Lumos has made serious progress toward that goal.
Key Developments Since Our Last Update
Aptatek Follow-On Contract (1 September 2025)
Lumos began the quarter by securing a US$1.5 million follow-on contract with Aptatek Biosciences to advance its in-home PKU monitoring device.
This deal expands Lumos’ service revenue base and showcases its growing reputation as a trusted development partner in rapid diagnostics, validation that the company’s expertise is in demand beyond FebriDx.
BARDA Paediatric Study (1 September 2025)
Soon after, the US government’s biomedical agency BARDA exercised its option to fund an additional US$6.2 million paediatric study.
This non-dilutive support extends FebriDx use to children aged 2–12, adding roughly 20% to the US market opportunity.
For investors, this marks further backing from a key US health authority and underscores confidence in Lumos’ technology and data.
YesGroup ANZ Distribution Deal (9 September 2025)
Lumos followed up with a second regional distribution agreement, signing YesGroup to complement existing partner Henry Schein.
This partnership strengthens local reach in Australia and New Zealand and builds early sales momentum ahead of the CLIA decision.
It reinforces Lumos’ ability to monetise FebriDx even before full US clearance.
Real-World Data From the UK (24 September 2025)
Later in the month, the NHS reported nearly two years of real-world FebriDx use. The data showed fewer antibiotics prescribed, fewer return visits, and measurable system cost savings.
This matters because it proves FebriDx not only works clinically but also drives economic efficiency. That's a powerful lever in future reimbursement negotiations. After all, the medical insurers are all about protecting their own hip pocket. So, funding for new treatments and diagnostics is often linked to the economic benefit to the insurer.
WellStreet Rollout Agreement (16 October 2025)
Most recently, Lumos announced its collaboration with WellStreet Urgent Care, a major US network partnered with Piedmont Healthcare in Atlanta.
Testing begins this month at one of WellStreet’s busiest clinics, expanding to around 140 locations nationwide if the CLIA waiver is granted.
This is Lumos’ first true step into real-world commercial deployment. It marks a massive indication of real demand in the market.
The Big Picture
From September through October, Lumos has ticked off milestone after milestone.
Between the Aptatek contract, BARDA’s funding, the YesGroup and WellStreet partnerships, and strong NHS validation, Lumos has evolved from a speculative biotech into a commercial stage diagnostics company with contracts, cash backing, and a clear growth pathway.
The demand for FebriDX is real, and the way we see it, the chance that CLIA is knocked back is extremely low.
Financial Position
Gross margin held steady at 63%, reflecting efficient manufacturing scale-up, while the EBITDA loss narrowed to US$3.4 million.
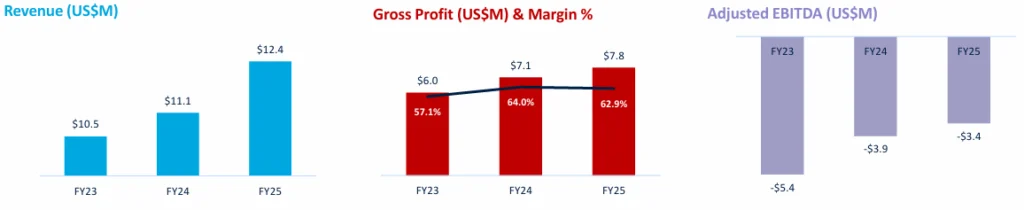
LDX FY25 Financial Highlights (Source: Lumos Diagnostics Company Presentation)
In our view, Lumos has enough cash and funding access to carry it through to the CLIA waiver decision.
The next raise will most likely occur once that decision is handed down, to fund the manufacturing ramp-up. If the CLIA waiver is granted, that capital raising should take place at a much higher share price than today.
Financially, Lumos looks clean, with no royalty burdens, no toxic convertibles, and a credible path to positive cash flow once FebriDx reaches broad US adoption.
Outlook
The FDA CLIA waiver decision is expected between November 2025 and February 2026. That’s the moment that opens the floodgates.
If the CLIA waiver is granted, US$5 million in prepaid orders from Phase Scientific will trigger immediately. This would also allow the national rollout through WellStreet’s 140 clinics to commence.
In addition, broader insurance coverage discussions, already underway via Pro-spectus, will accelerate as reimbursement pathways open up.
On top of that, the BARDA paediatric study will begin, expanding the clinical footprint, and the YesGroup partnership should start generating early ANZ sales.
In short, Lumos has multiple catalysts stacked for Q4 2025 through mid-2026.
This isn’t a blue-sky story anymore. It’s execution time.
Technical Analysis
After bottoming near 2-cents in mid-2025, LDX has built a strong ascending base, with a clear series of higher lows and higher highs.
The latest surge, triggered by the WellStreet deal, pushed the stock to test the pivotal 20-cent level. Unfortunately, it’s come amid general market weakness, and so we’ve seen the level hold for the time being.
Strong support rests at the 14-15 cent range.
If the CLIA waiver lands positive, we expect the 20-cent resistance level to be broken convincingly, with a move back to the target level of 60 cents in sight. We may see some noise around prior 27.5 and 33-cent consolidation areas.
A rejection of the CLIA waiver will vary in impact based on the form the rejection takes. We expect any rejection to most likely be a short-term negotiation that requires a re-submission within a few months.
However, on the slim chance that there’s some underlying issue with the actual data or the technology itself, a 50% plus sell-off is possible. With the blow being cushioned by the existing downside protection of the PHASE Scientific deal already in place.
It’s important to remember as well, that the company can still sell the test without the CLIA waiver. It’s just a much smaller sales opportunity.
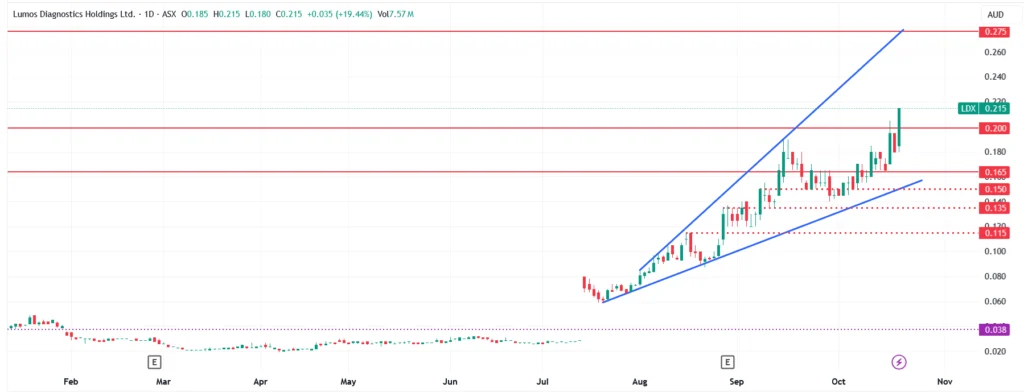
Lumos Diagnostics (ASX:LDX) Daily Price Chart (Source: TradingView)
Recommendation
The fundamentals are improving fast. The US rollout is underway. The CLIA decision window is approaching.
And the technical picture suggests the market is beginning to price that in.
This is still an early-stage, high-volatility play, but it’s backed by partnerships, government funding, and growing real-world data.
LDX remains one of the most asymmetrical setups in the biotech space right now. Limited downside at current levels, explosive upside if the waiver drops before Christmas.
Stay long, stay patient.
This one’s moving from proof-of-concept to real business right before our eyes.
This publication has been prepared by The Markets IQ, a division of Vitti Capital Pty Ltd (ABN 13 670 030 145), which is a Corporate Authorised Representative (001306367) of Point Capital Group Pty Ltd (ABN 41 625 931 900), the holder of Australian Financial Services Licence 518031. This report is for general information only and does not take into account your objectives, financial situation, or needs. It is not personal financial advice or a recommendation to buy, hold, or sell any security. You should consider whether the information is appropriate in light of your circumstances and obtain professional advice before making any investment decision. This report is intended solely for wholesale, sophisticated, or professional investors within the meaning of the Corporations Act 2001 (Cth).
Any views, probabilities, valuations, technical levels, or forecasts expressed are strictly the opinions of the authors as at the date of publication, based on publicly available information and assumptions which may change without notice. They are illustrative only and not predictive of future outcomes. Past performance is not a reliable indicator of future performance. Directors, staff, or clients of Vitti Capital may hold positions in Lumos Diagnostics (ASX:LDX) or related securities at the time of publication. Such holdings may change without notice. Vitti Capital applies internal controls to manage potential conflicts of interest; however, readers should assume that conflicts may exist.
The analyst(s) responsible for preparing this research note certify that the views expressed in this report accurately reflect their personal views about Lumos Diagnostics (ASX:LDX) and its securities. No part of their compensation is, or will be, directly or indirectly related to the specific recommendations or views expressed herein. The analyst(s) and/or their associates may hold an interest in Lumos Diagnostics (ASX:LDX).